McQueen Lab |
 |
Quantum Materials Research Group |
McQueen Lab |
 |
Quantum Materials Research Group |
Course Webpage: https://occamy.chemistry.jhu.edu/courses/AS.030.103/fall_2017/index.php
Last Updated: December 16, 2017
This course is designed for freshmen who have previously taken AP chemistry or have similar advanced chemistry experience. This course will review an advanced introductory chemistry sequence in a single semester. Chemical equilibrium, reactivity and bonding will be covered. These topics will be explored through the use of laboratory experiments and problem solving, and the use of these principles in current research areas will be discussed. Students may receive credit for AS.030.103 or EN.510.101, but not both.
At the end of this course, students will be able to:
| Class Times: | MWF 9:00-9:50 AM OR MWF 10:00-10:50 AM | |
| Lab Times: | M,T,W or Th 1:30-5:00 PM, or T 9:00-Noon | |
| Classrooms: | Monday/Wednesday | Bloomberg 478 (building 11 on the campus map) |
| Friday | Mergenthaler 111 (building 51 on the campus map) | |
| Labs | UTL G82/G84 (building 70 on the campus map) | |
| Final Exam | Shaffer 303/304 |
| INSTRUCTOR: |
| Prof. Tyrel M. McQueen |
| mcqueen@jhu.edu |
| Office: New Chemistry Building #312 and Bloomberg #301 |
| Office Hours:Tuesday, Noon-1 PM in NCB 312 or just stopping by ("open door policy") |
| TA Office Hours | ||
| Wednesdays, 6:30-7:30 PM | Remsen 347 | Mekhola, Saurov |
| Thursdays, 5:30-6:30 PM | Remsen 347 | Leslie, Veronica, Yue |
| Lab Day | TEACHING ASSISTANTS |
| Monday PM | Yue Shen (yshen45@jhu.edu) Kimberly Wang (kwang37@jhu.edu) |
| Tuesday AM | Veronica Stewart (vstewar4@jhu.edu) Hannah Korslund (hkorslu1@jhu.edu) |
| Tuesday PM | Leslie Sigmon (lsigmon2@jhu.edu) Melody (Tran) Nguyen (tnguy186@jhu.edu) |
| Wednesday PM | Saurov Hazarika (shazari1@jhu.edu) Lauren Bules (lbules1@jhu.edu) |
| Thursday PM | Mekhola Sinha (msinha4@jhu.edu) Adam Strickland (astrick4@jhu.edu) |
Point Distribution: 10% each of two hour exams, 14% final exam, 20% individual homework, 4% each of nine laboratory experiments (completion, notebook, and assignments), 5% in-class participation, 5% final project
Late Assignments: Lab and homework assignments will be marked off 10% for each day late. Assignments that are more than one week late will not be accepted. Late prelabs will not be accepted.
Final grades will be assigned by looking at class averages, medians, large point gaps between students, and comparisons to prior year cohorts. However, the following table indicates minimum grades awarded for a given percentage of points earned:
OVERALL COURSE GRADES: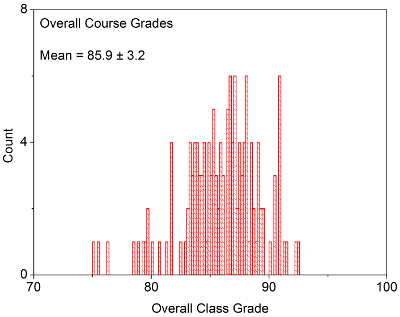
Please see TMM if you have any questions.
| Percentage | Minimum Grade |
| 90% | A- |
| 80% | B- |
| 70% | C- |
Regrade Policy: If you believe that a mistake was made in grading your exam, you may submit a regrade request. A regrade request should be a clean sheet of paper with a short description of what was done wrong paperclipped to the front of your exam. TMM reserves the right to inspect the exam for other grading errors if you submit a regrade request. All regrade requests are due by the dates that will be posted here. Submission of a formal regrade request is required to have your exam score changed, but TMM is of course available to discuss any issues or problems you have.
Excused Exams: If you are appropriately excused from an exam (see above), your class rank on each of the exams you took will be determined, and the average of this rank will be calculated. For the exam you missed, the numerical grade for the student of this rank will be entered.
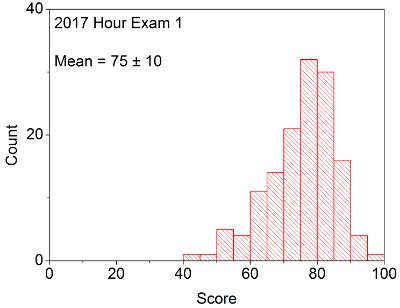
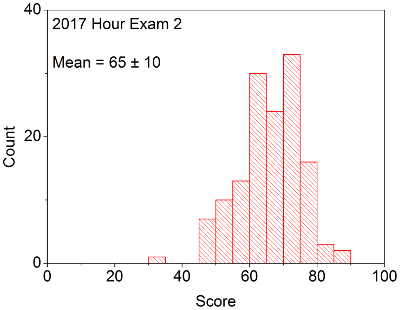
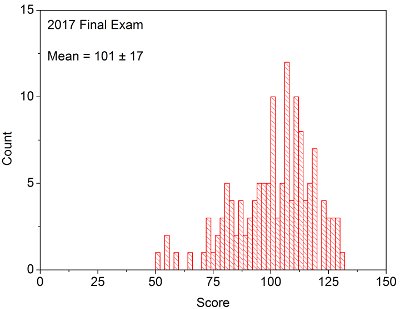
Your in-laboratory grade will be determined by the quality of your notes and observations as written down in your laboratory notebook. Laboratory notebooks are primary documentation and cannot be taken out of the laboratory, and instead will be turned into your TA at the conclusion of each laboratory period. You will be able to take the carbon copy of your notes in order to complete the associated problem sets / write ups.
The Care and Feeding of Your Lab Notebook (Lab Book Grading Sheet)
These are available on Sapling Learning via blackboard.
These will be posted on blackboard as required in the class.
| Class Week | Topic | Laboratory |
| Week 0 (8/31-9/1, M sch on Th): | Unit 1 (2 lectures) | No lab |
| Week 1 (9/5-9/8): | Unit 1 (2 lectures) | M: No Lab, TWTh: Lab A |
| Week 2 (9/11-9/15): | Unit 1 (2 lectures), Unit 2 (1 lecture) | M: Lab A, TWTh: Lab B |
| Week 3 (9/18-9/22): | Unit 2 (3 lectures) | M: Lab B, TWTh: Lab C |
| Week 4 (9/25-9/29): | Unit 2 (1 lecture), Unit 3 (1 lecture), EXAM 1 | M: Lab C, TWTh: Lab D |
| Week 5 (10/2-10/6): | Unit 3 (3 lectures) | M: Lab D, TWTh: Lab E |
| Week 6 (10/9-10/13): | Unit 3 (2 lectures), Unit 4 (1 lecture) | M: Lab E, TWTh: Lab F |
| Week 7 (10/16-10/19): | Unit 4 (2 lectures) | M: Lab F, TWTh: No Lab |
| Week 8 (10/23-10/27): | Unit 4 (2 lectures), EXAM 2 | MTWTh: Lab G1 |
| Week 9 (10/30-11/3): | Unit 5 (3 lectures) | MTWTh: Lab G2 |
| Week 10 (11/6-11/10): | Unit 5 (3 lectures) | MTWTh: Lab H |
| Week 11 (11/13-11/17): | Unit 5 (3 lectures) | MTWTh: Lab I |
| Thanksgiving Week (11/20-11/24) | ||
| Week 12 (11/27-12/1): | Unit 6 (3 lectures) | MTWTh: Lab SP and Checkout |
| Week 13 (12/4-12/8): | Unit 7 (3 lectures) | MTWTh: No Lab |
| 2-5 PM, Saturday, December 16th | Final Exam | Shaffer 303,304 |
These will be posted on blackboard as mentioned in the class.
Problem solving lecture slides are posted on blackboard after completion in class.
This course is not open for auditing.
You may add this course after the start of the semester. You must, however, contact TMM directly to make arrangements to make up any important work you may have missed.
If you are a student with a disability or believe you might have a disability that requires accommodations, please contact Dr. Brent Mosser in Student Disability Services, 385 Garland, (410)516-4720, studentdisabilityservices@jhu.edu
The strength of the university depends on academic and personal integrity. In this course, you must be honest and truthful. You may collaborate with other students in this course, but you must acknowledge this collaboration. Furthermore, you should collaborate with others rather than simply copying the ideas or solutions of others. Ethical violations include cheating on exams, plagiarism, reuse of assignments, improper use of the internet and electronic devices, unauthorized collaboration, alteration of graded assignments, forgery and falsification, lying, and facilitating academic dishonesty. For more information, see the guide on "Academic Ethics for Undergraduates" and the Ethics Board web site (http://ethics.jhu.edu).