McQueen Lab |
 |
Quantum Materials Research Group |
McQueen Lab |
 |
Quantum Materials Research Group |
Course Webpage: https://occamy.chemistry.jhu.edu/courses/AS.030.204/spring_2015/index.php
Last Updated: May 12, 2015
An introduction to the synthesis, structure, and reactivity of materials and inorganic compounds. Modern approaches to chemical bonding, including molecular orbital, ligand field, and crystal field theories, will be applied to understanding the physical and chemical properties of inorganic materials. Other topics to be discussed include magnetic properties, electronic spectra, magnetic resonance spectra, and reaction kinetics. The integrated laboratory will cover synthetic, measurement, and calculation methods of inorganic chemistry, and include hands-on exposure to state of the art materials research.
The material to be covered spans most chapters of the chosen text, with selected material from other resources as appropriate.
From Spring 2015 onward, this course also carries the writing-intensive designation.
| Class Times: | MWF 9:00-9:50 AM | |
| Lab Times: | M or Th 1:30-5:00 PM | |
| Classrooms: | Monday/Wednesday/Friday | Bloomberg 478 (building 10 on the campus map) |
| Labs | UTL G82/G84 (building 68 on the campus map) |
| INSTRUCTOR: |
| Prof. Tyrel M. McQueen |
| mcqueen@jhu.edu |
| Office: New Chemistry Building #312 and Bloomberg #301 |
| Office Hours:Tuesday, 1 PM to 2 PM in Bloomberg 301 |
| Office Hours This week: 5-7 PM, Wednesday, in UTL 289 |
| Lab Day | TEACHING ASSISTANTS | Location to turn Write Ups In |
| Monday | Jessica Panella (jpanell1@jhu.edu) | Gray Box in NCB 105 (building 14 on the campus map) |
| Kishan Patel | ||
| Thursday | Zachary Kelly (zkelly1@jhu.edu) | Gray Box in NCB 105 (building 14 on the campus map) |
| Harrison Charwat |
Point Distribution: 5% Watching Pre Lectures, 5% Pre Lecture Quizzes, 5% Class Participation, 25% Laboratory, 20% Laboratory Writeups/Problem Sets, 10% hour exam 2, 20% final exam, 10% Independent project lab and writeup
Late Assignments: Problem sets will be marked off 50% for each day late. Late Pre Lectures receive zero credit.
Extra Credit: Due to the cancelling of exam 1 due to inclement weather, you have an opportunity to complete the problems from that exam for extra credit (2.5%); answers are due by Thursday, March 26th, 9:00 AM in the box.
Final grades will be assigned by looking at class averages, medians, and large point gaps between students. However, the following table indicates minimum grades awarded for a given percentage of points earned:
| Percentage | Minimum Grade |
| 85% | A- |
| 75% | B- |
| 65% | C- |
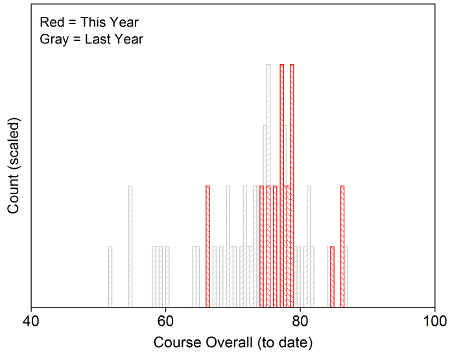
Above is the final distribution of scores for Spring 2015. The letter grade distribution was: 92% A+/A/A-, 8% B+/B/B- (justified by comparison to 2014).
Regrade Policy: If you believe that a mistake was made in grading your exam, you may submit a regrade request. A regrade request should be a clean sheet of paper with a short description of what was done wrong paperclipped to the front of your exam. TMM reserves the right to inspect the exam for other grading errors if you submit a regrade request. All regrade requests are due by the dates that will be posted here. Submission of a formal regrade request is required to have your exam score changed, but TMM is of course available to discuss any issues or problems you have.
Excused Exams: If you are appropriately excused from an exam (see above), your class rank on each of the exams you took will be determined, and the average of this rank will be calculated. For the exam you missed, the numerical grade for the student of this rank will be entered.
Your in-laboratory grade will be determined by the quality of your notes and observations as written down in your laboratory notebook. Laboratory notebooks are primary documentation and cannot be taken out of the laboratory, and instead will be turned into your TA at the conclusion of each laboratory period. You will be able to take the carbon copy of your notes in order to complete the associated problem sets / write ups.
The Care and Feeding of Your Lab Notebook (Lab Book Grading Sheet)
Lab Writeup Gradesheet (Example Writeup)
There are two kinds of homework assignments. Pre Lectures are short video lectures followed by an online quiz that are required to be completed by midnight prior to every class period. Problem Sets are associated with the laboratory experiments that you carry out and are due by 9 AM the following week (on the same day as your scheduled lab). If you have lab on Mondays, the questions from one lab are due by 9 AM the following Monday. If you have lab on Tuesdays, the questions from one lab are due by 9 AM the following Tuesday. If you have lab on Thursdays, the questions from one lab are due by 9 AM the following Thursday. Problem Sets are graded in the usual way, i.e. a number on a scale of 0 to 10. Pre Lectures are graded on an all-or-nothing (0 or 10 points) basis (for the purposes of grading any "passing" score on the Pre Lecture quiz will be awarded a full 10 points). If you do not pass the Pre Lecture quiz on the first attempt, you have an opportunity to watch a second (different) video lecture and have a second attempt at the problem(s), but only if the second attempt is also made before the deadline.
PROPOSAL DUE: Friday, April 10, 2015 at 09:00:00 AM (class)
PROPOSAL REVISIONS DUE: Monday, April 20, 2015 at 09:00:00 AM (class)
REPORT DUE: Friday, May 1, 2015 at 11:59:59 PM
REPORT REVISIONS DUE: Saturday, May 9, 2015 at 09:00:00 AM
Your independent project laboratory reports are due on Friday, May 1, at 11:59:59 PM. Reports will be accepted with no late penalty up to 11:59:59 PM on Sunday, May 3rd. You will receive feedback on your reports by end of day on Monday, May 4th. Revised reports are due on Saturday, May 9, at 09:00:00 AM (beginning of final exam period).
Pre Lectures and Problem Sets will be posted here as they are required for class. Pre Lectures will be posted no later than 5 PM on the date of the previous class (so a Wednesday prelecture link will appear here by 5 PM on Monday), but will sometimes be posted earlier. Lab handouts will typically be posted by 5 PM Friday of the previous week.
| Class Week | Topic | Laboratory | |
| Week 1 (1/26-1/30): | Coordination Chemistry: Structures and Isometers (3 lectures) | [M&T Ch. 9] | Safety and the Art of Measurement |
| Week 2 (2/2-2/6): | Crystal Fields and Spectrochemical Series (2 lectures), Effective Presentation of Scientific Data (1 lecture) | [M&T Ch. 4,10] | Spectrochemistry Lab |
| Week 3 (2/9-2/13): | Symmetry as an Organizing Principle (1 lecture), Symmetry Continued: Point groups (2 lectures) | [GER Ch. 4, M&T Ch. 4] | Symmetry Lab |
| Week 4 (2/16-2/20): | Symmetry Continued: Point Groups (2 lectures), Beyond CFT: MO Theory (1 lecture) | [M&T Ch. 5] | Quantum Dots Lab |
| Week 5 (2/23-2/27): | Beyond CFT: MO Theory (1.5 lectures), From MO Theory to Spectroscopy (1.5 lectures) | [M&T Ch. 11] | Kinetics Lab |
| Week 6 (3/2-3/6): | Solid State Structures (1 lecture) [RAINED OUT], Exam Review (1 lecture), HOUR EXAM 1 | [TMMHO] | No Lab (Exam Week) |
| Week 7 (3/9-3/13): | Introduction to Spectroscopy (2 lectures), Donor-Acceptor Chemistry (1 lecture) | [GER Ch. 5] | Solid State Synthesis Lab |
| Spring Break Week (3/16-3/20) | |||
| Week 8 (3/23-3/27): | Donor-Acceptor Chemistry (2 lectures), Inorganic Complex Reactivity (1 lecture) | [GER Ch. 6, M&T Ch. 6] | MOF Lab |
| Week 9 (3/30-4/3): | Inorganic Complex Reactivity (1 lecture), Effective Literature Searching (1 lecture), HOUR EXAM 2 | [M&T Ch. 12] | No Lab (Exam Week) |
| Week 10 (4/6-4/10): | Probing Reactions with Spectroscopy (1 lecture), From Reacitvity to Electrochemistry (2 lectures) | [A&J Ch. 14] | Redox/Electrochemistry Lab |
| Week 11 (4/13-4/17): | Electrochemistry (1 lecture) to Products and Magnetism (2 lectures) | [TMMHO] | Magnetic Properties Lab |
| Week 12 (4/20-4/24): | Inorganic and Solid State Reactivity | [TMMHO] | Independent Project 1 |
| Week 13 (4/27-5/1): | Bringing It All Together: Symmetry, Group Theory for Catalysis and Energy Conversion | [TMMHO] | Independent Project Continued |
| TBD | Final Exam (scheduled by registrar) | TBD | |
This course is not open for auditing.
You may add this course after the start of the semester. You must, however, contact TMM directly to make arrangements to make up any important work you may have missed.
If you are a student with a disability or believe you might have a disability that requires accommodations, please contact Dr. Brent Mosser in Student Disability Services, 385 Garland, (410)516-4720, studentdisabilityservices@jhu.edu
The strength of the university depends on academic and personal integrity. In this course, you must be honest and truthful. You may collaborate with other students in this course, but you must acknowledge this collaboration. Furthermore, you should collaborate with others rather than simply copying the ideas or solutions of others. Ethical violations include cheating on exams, plagiarism, reuse of assignments, improper use of the internet and electronic devices, unauthorized collaboration, alteration of graded assignments, forgery and falsification, lying, and facilitating academic dishonesty. For more information, see the guide on "Academic Ethics for Undergraduates" and the Ethics Board web site (http://ethics.jhu.edu).